KJ’s Story.
The future of personalized medicine coming at you.
“The first news summary of the morning —the most comprehensive I’ll receive all day— and the last thing I read before going on air.” — Hugh Hewitt, host of The Hugh Hewitt Show.
1. MIT News:
A protein located in the wrong part of a cell can contribute to several diseases, such as Alzheimer’s, cystic fibrosis, and cancer. But there are about 70,000 different proteins and protein variants in a single human cell, and since scientists can typically only test for a handful in one experiment, it is extremely costly and time-consuming to identify proteins’ locations manually.
A new generation of computational techniques seeks to streamline the process using machine-learning models that often leverage datasets containing thousands of proteins and their locations, measured across multiple cell lines. One of the largest such datasets is the Human Protein Atlas, which catalogs the subcellular behavior of over 13,000 proteins in more than 40 cell lines. But as enormous as it is, the Human Protein Atlas has only explored about 0.25 percent of all possible pairings of all proteins and cell lines within the database.
Now, researchers from MIT, Harvard University, and the Broad Institute of MIT and Harvard have developed a new computational approach that can efficiently explore the remaining uncharted space. Their method can predict the location of any protein in any human cell line, even when both protein and cell have never been tested before. (Source: news.mit.edu, italics mine)
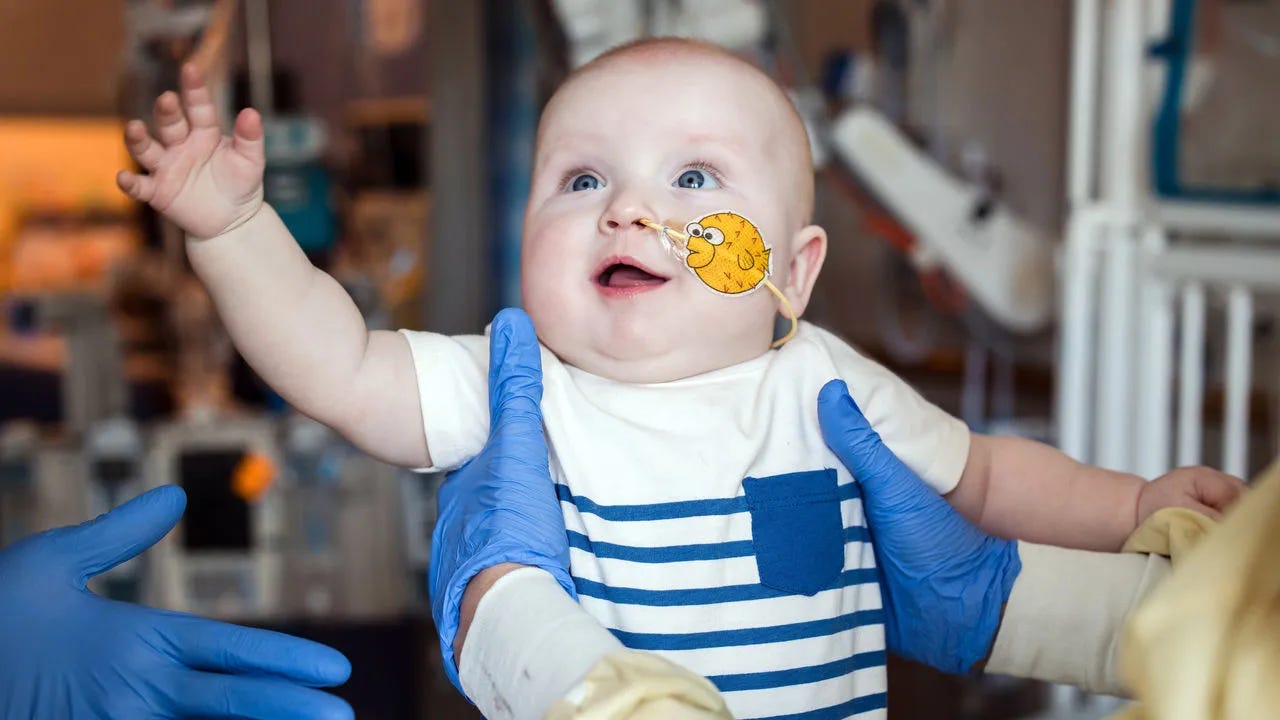
2. KJ:
(Photo Credit: Children’s Hospital of Philadelphia)
3. The Economist:
With days after KJ was born in Philadelphia in August 2024 it was clear that something was wrong. He was not eating and slept too much. Blood tests revealed sky-high levels of ammonia, a toxic substance the body usually expels. Genome sequencing confirmed that he had a rare genetic disease called carbamoyl-phosphate synthetase 1 (CPS1) deficiency, which often kills in infancy, and for which no good neonatal treatment exists. Then one of his doctors suggested something radical: a gene-editing drug designed specifically for him.
At face value, the idea was preposterous. Drug development takes years, time KJ did not have. But his doctor, Rebecca Ahrens-Nicklas, a metabolic-disease expert at the Children’s Hospital of Philadelphia and her colleague Kiran Musunuru, a geneticist from the University of Pennsylvania, believed they could produce a drug in months. Remarkably, their plan seems to have worked. KJ is now preparing to leave the hospital for the first time and go home to his family, after becoming the first person to be treated with a bespoke gene-editing therapy. This breakthrough could allow such treatments to one day become a routine option for children with debilitating genetic diseases. (Source: economist.com. The New England Journal of Medicine article on this is here.)